Pu Lab
The Pu lab studies cardiovascular development and disease. We seek to understand fundamental mechanisms that control normal cardiovascular development and function, and how they go awry in cardiovascular disease. Specific areas of interest include:
1. Gene regulation in cardiovascular development and disease.
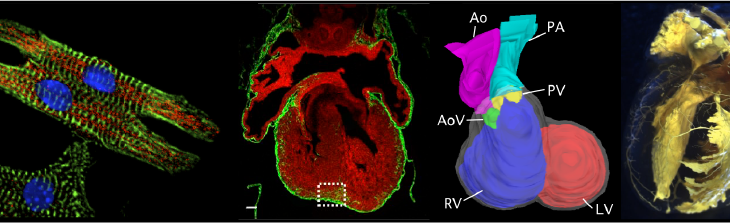
The development and function of the heart and vascular system is governed by transcriptional and epigenetic mechanisms, which regulate gene expression. Perturbation of these mechanisms cause congenital heart disease and vascular malformations. The Pu lab is investigating these gene regulatory mechanisms and their abnormalities in cardiovascular disease. To achieve these goals, we are using both genetically engineered mice and induced pluripotent stem cells. We combine genome engineering with CRISPR/Cas9, genome-wide epigenetic and transiptional profiling, single cell RNA-sequencing, and other cutting edge approaches to develop rigorous mechanistic insights.
Representative publications:
- He, A., Kong, S. W., Ma, Q. & Pu, W. T. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc. Natl. Acad. Sci. U. S. A. 108, 5632–5637 (2011).
- Zhou, P., Gu, F., Zhang, L., Akerberg, B. N., Ma, Q., Li, K., He, A., Lin, Z., Stevens, S. M., Zhou, B. & Pu, W. T. Mapping cell type-specific transcriptional enhancers using high affinity, lineage-specific Ep300 bioChIP-seq. Elife 6, (2017).
- Akerberg BN, Gu F, VanDusen NJ, Zhang X, Dong R, Li K, Zhang B, Zhou B, Sethi I, Ma Q, Wasson L, Wen T, Liu J, Dong K, Conlon FL, Zhou J, Yuan G-C, Zhou P, Pu WT. A reference map of murine cardiac transcription factor chromatin occupancy identifies dynamic and conserved enhancers. Nat Commun. 2019 Oct 28;10(1):4907.
2. Understanding disease mechanisms in pediatric heart disease.
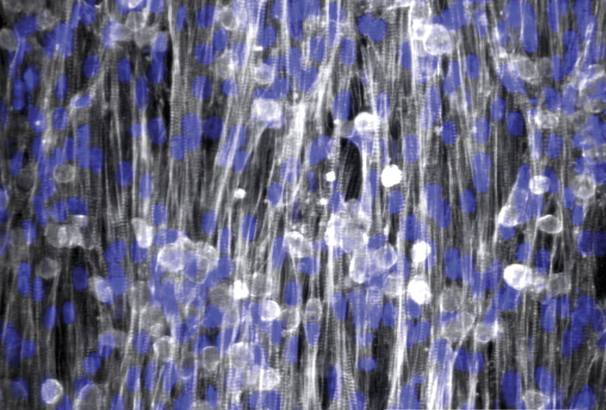
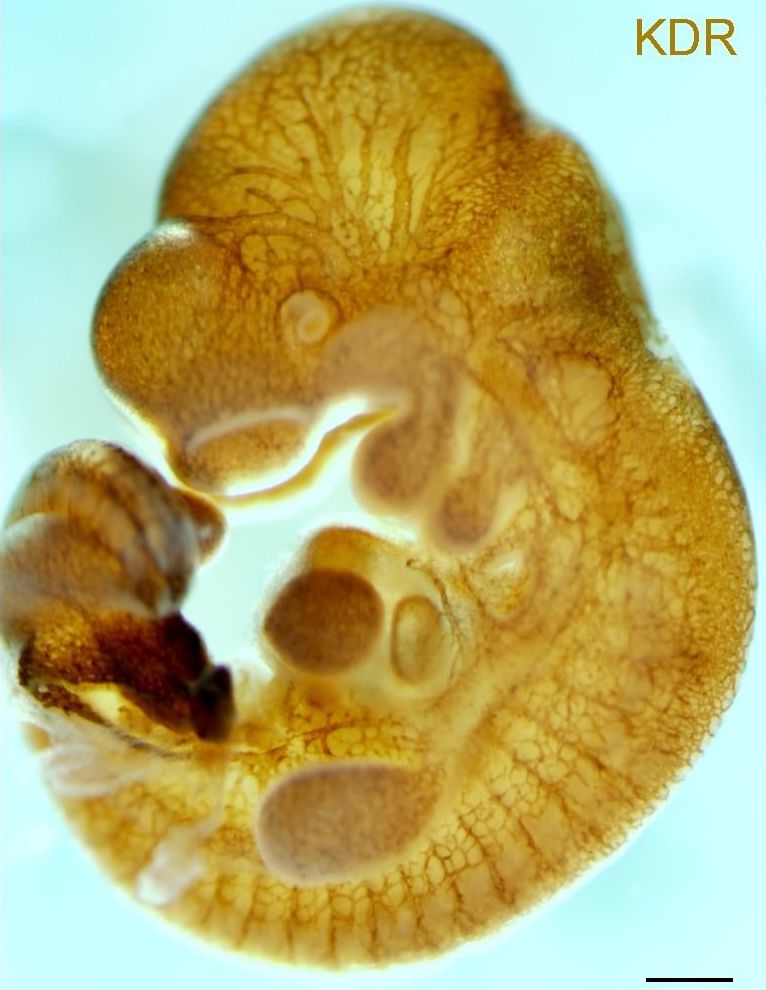
The lab uses both induced pluripotent stem cells and mice to develop models of heart diseases seen in our Pediatric Cardiology patients. We use these models to develop cellular and molecular insights into disease pathogenesis. These insights allow us to develop novel targeted therapies. Among the diseases we are studying are Barth syndrome, a mitochondrial myopathy, CPVT, an inherited arrhythmia, and Arrhythmogenic Cardiomyopathy, which causes both heart muscle weakness and arrhythmia.
Representative publications:
- Wang, G., McCain, M. L., Yang, L., He, A., Pasqualini, F. S., Agarwal, A., Yuan, H., Jiang, D., Zhang, D., Zangi, L., Geva, J., Roberts, A. E., Ma, Q., Ding, J., Chen, J., Wang, D. Z., Li, K., Wang, J., Wanders, R. J., Kulik, W., Vaz, F. M., Laflamme, M. A., Murry, C. E., Chien, K. R., Kelley, R. I., Church, G. M., Parker, K. K. & Pu, W. T. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 20, 616–623 (2014).
- Liu X, Wang S, Guo X, Li Y, Ogurlu R, Lu F, Prondzynski M, de la Serna Buzon S, Ma Q, Zhang D, Wang G, Cotton J, Guo Y, Xiao L, Milan DJ, Xu Y, Schlame M, Bezzerides VJ, Pu WT. Increased ROS-mediated CaMKII activation contributes to calcium handling abnormalities and impaired contraction in Barth syndrome. Circulation. 2021 in press.
- Park S-J, Zhang D, Qi Y, Li Y, Lee KY, Bezzerides VJ, Yang P, Xia S, Kim SL, Liu X, Lu F, Pasqualini FS, Campbell PH, Geva J, Roberts AE, Kleber AG, Abrams DJ, Pu WT*, Parker KK*. Insights Into the Pathogenesis of Catecholaminergic Polymorphic Ventricular Tachycardia From Engineered Human Heart Tissue. Circulation. 2019 Jul 30;140(5):390–404. *co-corresponding
3. Development of novel therapies for pediatric heart disease.
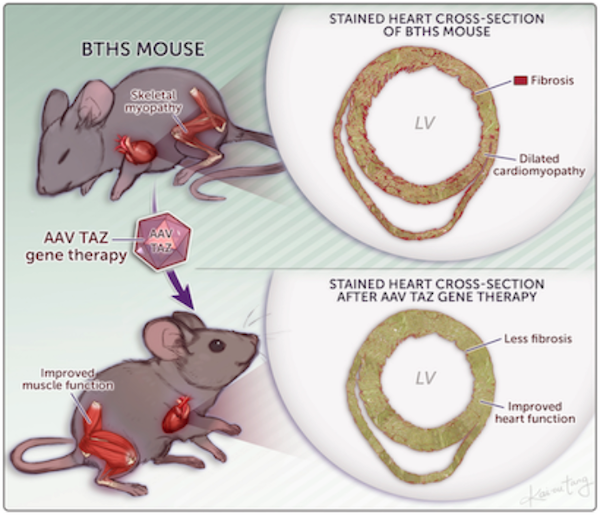
The lab exploits insights into mechanisms underlying inherited heart disease to develop novel therapies. One particularly exciting therapeutic avenue for inherited heart disease is adeno-associated virus (AAV) delivery of therapeutic genetic cargo to heart muscle cells.
Representative publications:
- Bezzerides VJ, Caballero A, Wang S, Ai Y, Hylind RJ, Lu F, Heims-Waldron DA, Chambers KD, Zhang D, Abrams DJ, Pu WT. Gene Therapy for Catecholaminergic Polymorphic Ventricular Tachycardia by Inhibition of Ca2+/Calmodulin-Dependent Kinase II. Circulation. 2019 Jul 30;140(5):405–19.
- Wang S, Li Y, Xu Y, Ma Q, Lin Z, Schlame M, Bezzerides VJ, Strathdee D, Pu WT. AAV Gene Therapy Prevents and Reverses Heart Failure in a Murine Knockout Model of Barth Syndrome. Circ Res. 2020 Apr 10;126(8):1024–39.
For more information, visit the Pu Lab website.
William Pu

William Pu, MD, is the Director of Basic and Translational Cardiovascular Research in the Department of Cardiology at Boston Children’s Hospital, and the Aldo R. Professor of Pediatrics at Harvard Medical School. Dr. Pu has broad expertise in cardiac biology that includes cardiac development, heart failure, cardiac regeneration and in vitro cardiac disease modeling. His lab has made fundamental discoveries in gene regulation in developing and diseased hearts, particularly in the area of transcriptional regulation. Many of these discoveries are the result of innovative approaches to studying heart development and disease, often involving multidisciplinary collaborations that draw on advances in other fields. His lab is currently engaged in research projects on the genetic causes of inherited heart diseases, and gene therapy for Barth Syndrome and CPVT.
Dr. Pu has Dr. Pu completed his combined BS-MS degree at Yale University and obtained his MD degree from the Harvard Medical School/MIT Science and Technology Program in 1993. He trained in Pediatrics and Pediatric Cardiology at Boston Children’s Hospital. He received his training in basic research in the laboratories of Kevin Struhl, David Clapham, and Seigo Izumo. He established an independent research lab at Boston Children’s in 2004.
Contact Dr. Pu at wpu@pulab.org.