Wang Lab
The Wang Lab is interested in the molecular mechanisms that control cellular differentiation and morphogenesis during animal development. We use muscle cells as our model system and we apply a variety of molecular, cellular, biochemistry and genetic approaches, including transgenic and knockout mouse models in our lab. Current areas of research include:
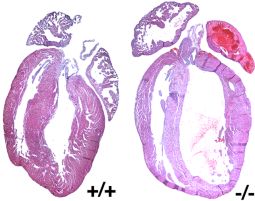
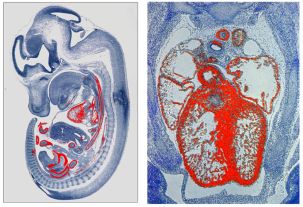
1. Transcriptional and epigenetic control of mammalian cardiovascular development and function.
Our studies focus on the identification of cell growth and differentiation signals that modulate the transcriptional activities of myocardin family of transcription factors. We are also investigating how pathophysiological signals, such as cardiac hypertrophy and congenital heart failure, mediate change in myocardin-dependent cardiac gene expression. In addition, we study how epigenetic factors, which modify chromatin, impact cardiac transcription factors and cardiac gene expression during cardiovascular development.
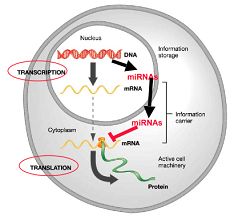
2. miRNAs in cardiac muscle development and function.
To date, more than 700 human miRNAs have been identified. It has been estimated that at least 1% of the transcripts in the genome code for miRNAs, and that approximately 50% of all human genes are targets of miRNA regulation. However, the molecular mechanisms and the in vivo functions of most miRNAs remain unknown.
The long-term goal of our research is to understand the molecular mechanisms in which miRNAs control the proliferation and differentiation of cardiac muscle. We are currently investigating the in vivo function of miRNAs in cardiac muscle using both gain- and loss-of-function approaches via transgenic and gene knockout technologies.
3. miRNAs in skeletal muscle stem cell and regeneration.
Skeletal muscle satellite cells are adult stem cells responsible for postnatal skeletal muscle growth and regeneration. Satellite cells, which normally remain mitotically quiescent, will be activated in response to growth and stress signals to give rise to myogenic progenitor cells. Whereas the transcriptional events and signaling pathways that control satellite cell fate and function have been extensively investigated, the potential involvement of miRNAs in satellite cells has not been explored. We hypothesize that miRNAs are required for proper satellite cell fate determination and self-renewal. We have performed miRNA expression profiling analysis in satellite cells and in skeletal muscle myoblasts and have identified a dozen interesting miRNAs expressed in satellite cells. We will investigate how those miRNAs regulate the self-renewal, proliferation and differentiation of those cells. Our studies will provide insights into the molecular mechanisms of stem cell biology and regenerative medicine.
For more information, visit the Wang Lab Website.

Dr. Da-Zhi Wang received his Ph.D. in 1998 from the Department of Biological Sciences of the University of Iowa in the laboratory of Prof. Jim Lin where he studied vertebrate development. Dr. Wang conducted his postdoctoral training in the laboratory of Prof. Eric Olson at the University of Texas Southwestern Medical Center at Dallas from 1998 to 2002. As a postdoctoral fellow and instructor, Dr. Wang identified a novel transcription factor, myocardin, and demonstrated that myocardin is essential for cardiovascular development. In 2002, Dr. Da-Zhi Wang was recruited to UNC-CH as an Assistant Professor of the Department of Cell and Developmental Biology and a member of the Carolina Cardiovascular Biology Center (CCBC) to establish his independent research program. He was promoted to Associate Professor with tenure in 2008 at UNC. Dr. Wang was recruited to the Division of Cardiovascular Research of Children’s Hospital Boston and Harvard Medical School in July 2009 and relocated his lab from Chapel Hill to Boston.