Manuscript accepted — Engineered human tissue model of CPVT
Full text available here.
In an equal collaboration between the Pu and Parker labs, Park, Zhang, et al. describe development of an engineered human tissue model of CPVT. Using this model, the investigators dissected the molecular pathway linking exercise to unmasking of the arrhythmia mutation. The study revealed that CaMKII phosphorylation of RYR2-S2814 is essential for expression of multiple RYR2 mutations.
BACKGROUND:
Modeling of human arrhythmias using induced pluripotent stem cell-derived cardiomyocytes has focused on single cell phenotypes. However, arrhythmias are the emergent properties of cells assembled into tissues, and the impact of inherited arrhythmia mutations on tissue-level properties of human heart tissue has not been reported.
METHODS:
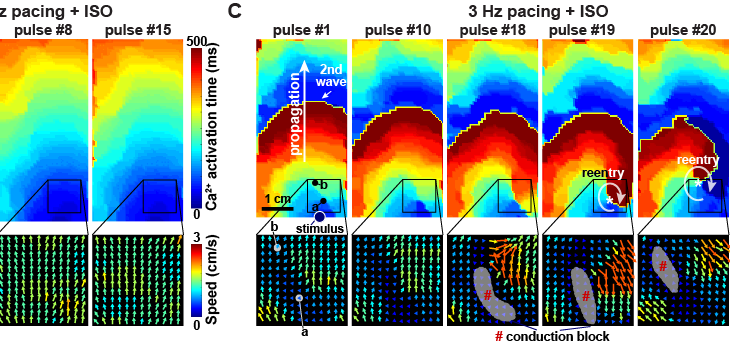
Here, we report an optogenetically-based, human engineered tissue model of catecholaminergic polymorphic ventricular tachycardia (CPVT), an inherited arrhythmia caused by mutation of the cardiac ryanodine channel (RYR2) and triggered by exercise. We developed a hiPSC-CM-based platform to study the tissue-level properties of engineered human myocardium. We investigated pathogenic mechanisms in CPVT, by combining this novel platform with genome editing.
RESULTS:
In our model, CPVT tissues were vulnerable to develop reentrant rhythms when stimulated by rapid pacing and catecholamine, recapitulating hallmark features of the disease. These conditions elevated diastolic Ca2+ levels and increased temporal and spatial dispersion of Ca2+ wave speed, creating a vulnerable arrhythmia substrate. Using Cas9 genome editing, we pinpointed a single catecholamine-driven phosphorylation event, RYR2-S2814 phosphorylation by Ca2+-calmodulin-dependent protein kinase II (CaMKII), that is required to unmask the arrhythmic potential of CPVT tissues.
CONCLUSIONS:
Our study illuminates the molecular and cellular pathogenesis of CPVT and reveals a critical role of CaMKII-dependent reentry in the tissue-scale mechanism of this disease. We anticipate that this approach will be useful to model other inherited and acquired cardiac arrhythmias.